BIOPHARMACEUTICAL DEVELOPMENT
Over ten years of experience in biologic medicines
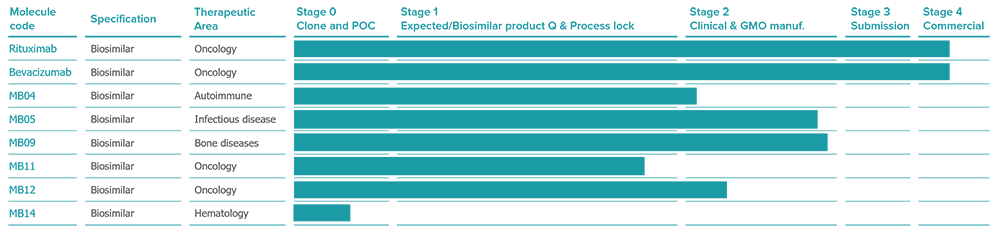
PIPELINE
A growing portfolio to increase patient access to high quality treatments
We are specialists in biologics: we have developed and commercialized two biosimilar medicines for oncology, available to thousands of patients across the globe via our B2B partnerships in worldwide markets: MB01 rituximab biosimilar and MB02 bevacizumab biosimilar, since 2014 and 2016 respectively. Now we have a highly attractive pipeline of biosimilar and innovative biopharmaceuticals targeting areas of oncology, hematology, osteoporosis and pediatrics.
Our development of biopharmaceuticals adheres to a strict “quality by design” standard using the latest innovations in upstream, downstream and cutting edge-analytics. We guarantee all biosimilars are equivalent in terms of quality, safety and efficacy to the reference products, by subjecting the drugs to exhaustive comparability testing and clinical trials, regulated by the competent authorities.
RESEARCH & DEVELOPMENT
In-house R&D competencies set apart our development of biopharmaceuticals
Our highly experienced team has specialist knowledge of chemistry and biochemistry related to biopharmaceuticals. Thanks to our in-house R&D platform, we ensure maximum control over quality and delivery of development programs.
PARTNERSHIPS
Our strategic alliances give us access to 100+ markets across the globe
We have established alliances with key partners, granting patients access to our quality medicines across the globe. Currently, we have a presence in over a hundred markets worldwide through network of over 30 global and regional partners, and we focus on structuring strategic partnerships for new products across the regulated markets.
At mAbxience, we work to ensure supply of affordable medicines to health care systems worldwide, adapting to the needs of each regulatory and commercial environment. We are committed to providing excellence for all our partners — we achieve this by our world-class manufacturing, a mature and efficient supply chain and the flexibility to adapt to the commercial challenges faced by each market.
























